
perpendicular to the beam) can be calculated from The magnitude Vy of the velocity acquired in a direction parallel to the field ( i.e. 3.5), the electric field produced a force (and therefore an acceleration a) on each particle over a distance L, deflecting the beam through an By adjusting the strength of one of the fields, so as to produce zero net deflection as observed on a fluorescent screen, he achieved the condition:Īnd so was able to measure the speed Vx of the particles in the beam (travelling in the x-direction). The two fields were perpendicular to the beam and to each other, such that the magnetic and electrical forces were both perpendicular to the beam but opposite in direction. (between two parallel plates) and a magnetic field B (produced by an electromagnet) acting over the same region of space
Thomson passed a beam of cathode rays through a uniform electric field E Low-pressure gas was known to produce cathode rays, which could cause an object in their path to emit light ( fluoresce), but it was not known if these rays consisted of waves or material particles. The properties of electrons were investigated by J.J.Thomson at the Cavendish Laboratory in 1897 Although its interpretation was not clear at the time, Faraday's law reflects the fact that electricity passes through an electrolyte in the form of ions whose mass is proportional to the atomic weight and whose charge is equal to the valence, which represents the number of electrons which have been removed from the neutral atom. Has flowed around the circuit (current times time) and to the atomic weight of that element, but inversely proportional to the valence of the element. Faraday's law states that when a current is passed through a solution or through a molten electrolyte, the mass of a particular element deposited at the cathode or anode is proportional to the electrical charge which
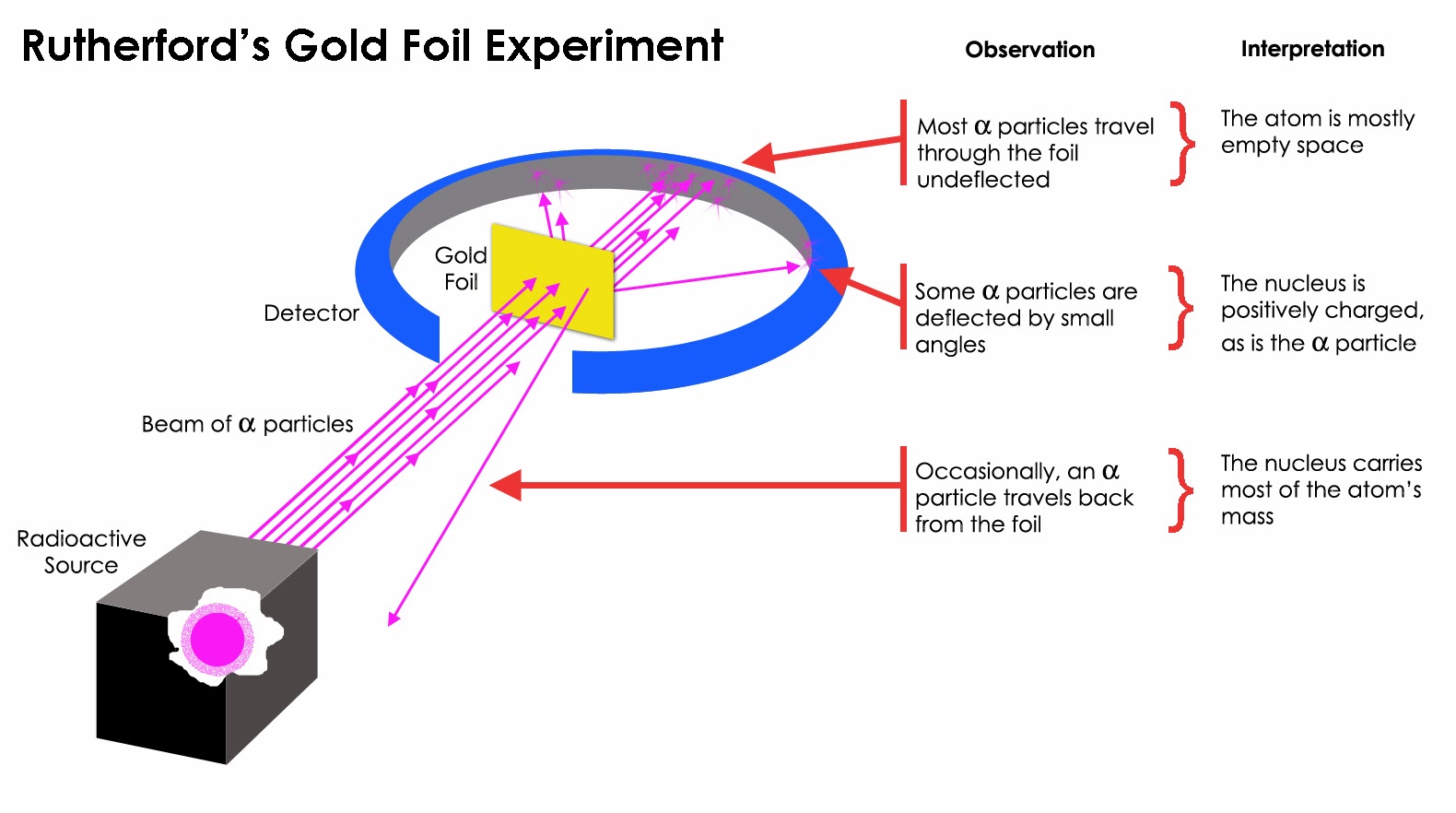
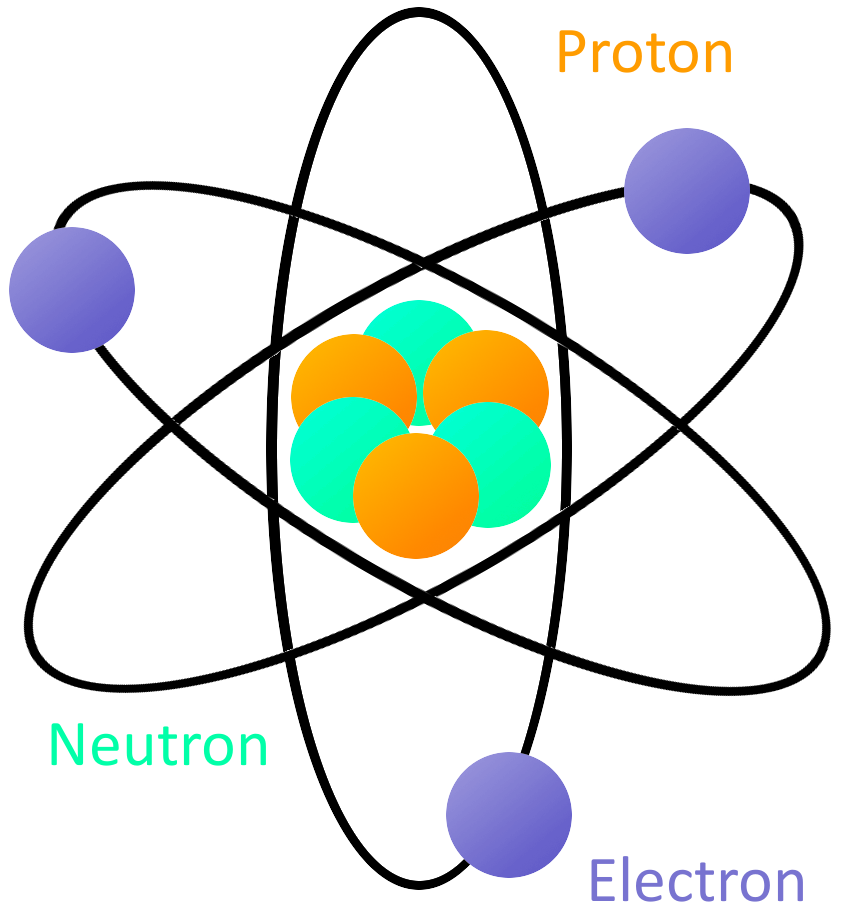
Other evidence came from chemistry, such as the experiments of Faraday on electrolysis (1833). The first direct evidence came from observations of the Brownian motion. Figure references are to the second edition of Modern Physics by Serway, Moses and Moyer (Saunders, 1989).Īlthough some ancient Greeks (such as Democritus) postulated the existence of atoms (units of matter which could not be subdivided), concrete evidence for their existence did not develop until the 19th century.


 0 kommentar(er)
0 kommentar(er)
